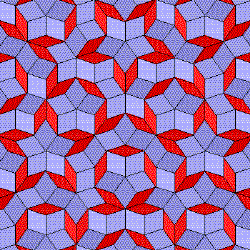
This is called a Penrose Tiling. This demonstrates the aperiodic layout of repeated tiling which gives an artistic visual of the quasicrystal.
Dan Schechtman discovered the "impossible" crystalline structure over 20 years ago in a lab. In April of 1982, Schechtman had rapidly chilled a molten mix of aluminum and manganese expecting to observe complete disorder at the atomic level. Instead, he saw a crystal, except, it was one that did not make any sense.
It is important to note that the paradigm at the time was that crystals existed in limited numbers of rotation symmetry: 1, 2, 3, 4 & 6 fold. Not 5, and not greater then 6.
Quick vocabulary break down:
Crystals - usually, when atoms are arranged in a way which is periodic
Rotation Symmetry - When a shape or image can be rotated and it still looks the same. For 4-fold symmetry, for example, if you rotate the image four times, it looks the same each time (a square is of four fold symmetry).
Paradigm - a constant based not on theory but observation.
How the structure of an atom is observed: shine a monochromatic (or single wave-length of) xrays on a specimen. That beam is diffracted by the atoms and displays a pattern on the other side. This is where the symmetry number is revealed.
This is how an electron microscope works. What Schechtman saw was a diffraction pattern of electrons on a t.v. scanner...
What Schechtman did with his aluminum-manganese mix was observe the diffraction using an electron microscope and that diffraction pattern displayed a crystal with five-fold symmetry. It went against the paradigm which had existed since 1912! He quickly ruled out "twinned" atoms, or atoms which would have a mirror image in symmetry. What was significant about five-fold symmetry was that it produces a pattern that cannot be repeated; it takes the "periodic" out of the crystalline structure.
Schechtman was ridiculed by his peers for years, and he was even kicked out of his research group when he refused to back down on his findings.
Over the years, Schechtman's findings were slowly accepted into the scientific community and applied to modern technology, making stainless steel stronger (especially for small tools and instruments such as electric razors and surgical tools) and surfaces slicker. Quasicrystals have even been found to naturally occur in minerals found in a Russian river.
In her book, Lisa Randall explains the significance of quasicrystals to scientific theories that require extra spacial dimensions: "Quasicrystals are fascinating structures whose underlying order is revealed only with extra dimensions." As in, that periodical structure that can't be found in quasicrystals in three dimensions, may be, while not observable (by us), possible in extra dimensions of space. This would help to understand that non-stick pan coating: "The nonstick frying pans that are coated with quasicrystals exploit the structural differences between the projections of higher-dimensional crystals in the pan's coating and the more mundane structure of ordinary three-dimensional food."
Dan Schechtman's discovery resulted in some fantastic theory support as well as important applications. It is well deserving of a Nobel Prize. Congratulations, Prof. Schechtman!
References:
Randall, Lisa. Warped Passages Unraveling the Mysteries of the Universe's Hidden Dimensions. Harper Perennial. 2005.
"The Nobel Prize in Chemistry 2011 - Popular Information". Nobelprize.org. 10 Oct 2011 http://www.nobelprize.org/nobel_prizes/chemistry/laureates/2011/info.html
Technion Institue - Interview with Prof. Dan Shechtman.
http://www.youtube.com/watch?v=EZRTzOMHQ4s